标签:
+Author Affiliations
Objectives. In order to allow personalized medicine, adequate prediction of disease outcome is required. In early undifferentiated arthritis (UA), prediction of the development of RA is crucial, and in case of RA predicting the severity of the disease course may guide individualized treatment decisions.
Methods. A total of 570 UA patients and 676 RA patients included in the Leiden Early Arthritis Clinic cohort were studied for baseline characteristics. The disease outcomes studied were fulfilment of the 1987 ACR-RA criteria and arthritis persistence in UA patients and the rate of radiological joint destruction and achieving sustained DMARD-free remission in RA patients.
Results. Predictive factors for fulfilment of the 1987 ACR-RA criteria and for persistent arthritis in UA were largely similar. Risk factors for a severe rate of joint destruction were: older age (P < 0.001); male gender (P < 0.001); longer symptom duration at first visit (P = 0.048), involvement of lower extremities (P < 0.001); BMI (P < 0.001); high acute phase reactants, presence of IgM-RF (P < 0.001); anti-CCP2 antibodies (P < 0.001); anti-modified citrullinated vimentin antibodies (P < 0.001) and HLA-DRB1 shared epitope alleles (P = 0.001). A high BMI was associated with a lower rate of joint destruction but with a higher risk of disease persistence. The proportion of variance in joint destruction explained was 32%
Conclusion. Predictors for RA development, previously used to develop a prediction rule in UA patients, are largely similar to predictors for arthritis persistency. Only part of the joint destruction level in RA is explained by the currently known risk factors. New factors need to be identified in order to guide pharmaceutical intervention at the level of individual RA patients.
The outcome of early arthritis patients is highly variable. Approximately only one-third of the patients with a recent-onset undifferentiated arthritis (UA) progress to RA. The severity of the progression of joint destruction in RA is highly variable as well, as only a minority will become severely destroyed. In order to achieve individualized treatment decision-making, the severity of the disease outcome needs to be estimated adequately. This is particularly relevant since it is widely acknowledged that early initiation of treatment of RA is effective in diminishing the level of joint destruction and disability [1–3]. Fewer studies are performed on the effects of early intervention in recent-onset UA, but available data suggest that early treatment strategies hamper progression in UA as well [4–6]. Potent treatment strategies such as targeted therapies are generally not started in an early phase because of the risk of overtreatment. However, when the individuals who will have an unfavourable disease outcome can be identified at first presentation, the risk of overtreatment and undertreatment can be balanced, resulting in a personalized pharmaceutical regimen.
Observational studies of unselected patients are most appropriate to identify risk factors for a certain disease course. Following patients with and without risk factors allows direct assessments of absolute risks of a disease outcome. The Leiden Early Arthritis Clinic (EAC) cohort is a population-based inception cohort including early arthritis patients since 1993. Patients are being followed as long as they are seen at the rheumatologist and follow-up ends when patients are discharged because of having a sustained DMARD-free remission or when patients die. During the past years, several risk factors for a mild or progressive disease course, both in UA and RA, have been identified.
The present article on this themed issue on registries in rheumatological conditions reviews to what extent the disease outcome in early UA and early RA can be predicted, using data from the Leiden EAC cohort. The two disease outcomes studied in UA include fulfilling the 1987 ACR criteria for RA and having persistent arthritis. The disease outcomes studied in early RA patients are the progression in joint destruction over time and disease persistence. These evaluations allow comparison of risk factors for joint destruction and RA persistency. Since it is thus far unclear to what extent the processes underlying joint destruction are similar to the processes that mediate disease persistency, evaluation of overlapping and dissimilar risk factors may increase understanding and the subsequent elucidation of the underlying biological pathways leading to these phenotypic characteristics. Finally, the fraction of explained variance of progression in joint destruction by the currently known risk factors is determined to assess how complete our current understanding is.
This Leiden EAC is a population-based prospective cohort that was started in 1993 in order to detect and treat inflammatory disorders early in the disease state, especially early RA. In order to obtain early referrals by general practitioners (GPs), a campaign was started among GPs to refer patients with suspected arthritis as soon as possible to the rheumatology department of the Leiden University Medical Center. This is the only centre for rheumatic diseases in a semi-rural area with >400 000 inhabitants. Patients are seen within 2 weeks. Inclusion took place when arthritis was confirmed at physical examination and symptom duration was <2 years. Written informed consent was obtained from all participants. The study was approved by the local medical ethics committee (ethics committee of the Leiden University Medical Center). At the first visit, the rheumatologist completed a questionnaire regarding the presenting symptoms, as reported by the patient: type, localization and distribution of initial joint symptoms, symptom duration and course of the initial symptoms. The patient’s smoking history and family history were assessed. Patients rated morning stiffness on a visual analogue scale (VAS; range 0–100 mm); the duration of morning stiffness was also assessed. The HAQ was used to provide an index of disability. A 66-joint count for swollen joints (SJC) was performed. Blood samples were taken for routine diagnostic laboratory screening [including CRP, ESR and immunoglobulin (Ig)M-RF] and stored to determine other serum markers (among other antibodies against citrullinated peptide antibodies) at a later time. Blood samples were taken for DNA extraction as well. Follow-up visits with standard clinical assessments (including an SJC and a HAQ) were performed 3 months after the first presentation and yearly thereafter. Radiographs of the hands and feet were taken at baseline and yearly thereafter. Two weeks after inclusion, when results of laboratory investigations and radiography were known, patients who had a form of arthritis that could not be classified according to ACR (formerly, the ARA) criteria were documented as having UA. The diagnosis of RA was established in cases where patients fulfilled the 1987 ACR criteria for RA. The initial treatment of RA patients had changed in time and differed according to the inclusion period [Knevel et al. (2010, data not published)]. Patients included between 1993 and 1995 were initially treated with analgesics and were subsequently treated with HCQ or SSZ if they had persistent active disease. Between 1996 and 1998, patients who were included were promptly treated with chloroquine or SSZ, whereas after 1998, the initial treatment strategy consisted of either MTX or SSZ [Knevel et al. (2010, data not published)]. Treatment of UA patients was not protocolized.
Patients with UA were assessed on two outcomes. First, after 1 year of follow-up, the fulfilment of the 1987 ACR criteria for RA was evaluated. As previously described, 31% of UA patients progressed to RA during 1 year of follow-up. The majority of the patients (94%) had been followed up for >1 year [mean follow-up (S.D.) 8 (3) years] and 4.4% of UA patients developed RA >1 year after inclusion [7]. The second disease outcome was disease persistence. As a generally accepted definition of persistence is lacking, we defined persistent arthritis as the absence of sustained DMARD-free remission. Sustained remission was diagnosed when patients had no swollen joints for at least 1 year after cessation of eventual DMARD therapy. The absence of swollen joints had to have been observed by a rheumatologist for at least 1 year to ensure that remission was not temporary, but rather sustained. When remission was not obtained after 5 years of disease, a patient was classified as having persistent disease in the present study.
Patients with RA were studied for the rate of radiological joint destruction and for achieving sustained DMARD-free remission or having persistent RA and also during a 5-year period of follow-up. In order to study the progression rate in a sensitive way, all serial radiographs were scored by one experienced reader (M.P.M.vd.L.) according to the Sharp–van der Heijde score (SHS) in chronological order. Four hundred and nine radiographs belonging to 60 randomly selected RA patients were rescored. The intra-class observer correlation coefficient was 0.91 for all scored radiographs and 0.97 for the radiographic progression rate. The means (S.E.M.) at the subsequent time points were 9.15 (0.43) at baseline; 15.65 (0.72) at 1 year follow-up; 20.0 (0.93) at 2 years; 24.79 (1.36) at 3 years; 34.83 (2.14) at 4 years; and 34.8 (2.14) at 5 years of follow-up. Persistent RA was defined as the absence of a sustained DMARD-free remission. A sustained DMARD-free remission in RA was defined as the absence of swollen joints for at least 1 year after cessation of DMARDs and classification as DMARD-free remission by the rheumatologist. To ensure that remission was not temporary but rather sustained and long lasting, the absence of swollen joints had to have been observed by a rheumatologist for at least 1 year after discontinuation of DMARD therapy. Corticosteroids were considered here to be equivalent to DMARDs. The majority of patients with disease in remission were discharged from the outpatient clinic at any time; however, most patients who achieved remission were followed up longer than the minimum requirement of 1 year; the median time of observation after discontinuation of DMARDs in the absence of swollen joints was 2.5 years. Patients who had a recurrence of their arthritis after discharge could easily return to the Leiden University Medical Center. The frequency of disease relapse was 6%; these patients were included in the persistency group. We observed previously that sustained DMARD-free remission was obtained by 15% of RA patients after a median disease duration of 43 months [8]. Therefore, for the present study, those patients who did not achieve a sustained DMARD-free remission within the first 5 years were classified as having persistent RA.
Predictors for RA development and arthritis persistency were analysed univariately with a logistic regression analysis. Since the aim of the present study was to review predictive factors and not to develop a prediction rule for the outcome of UA, which has been done before [9], no multivariate regression analysis was performed in the UA patients.
Associations between baseline factors and rate of joint destruction were analysed with a linear multivariate regression model (see Knevel et al., 2010, manuscript under review for a detailed description). This was done for each variable separately, but all analyses were adjusted for the applied treatment strategy. In a previous study, we showed that the inclusion period is an adequate proxy for the different treatment strategies that were applied over time (Knevel et al., 2010, manuscript under review). The baseline characteristics were tested with an interaction term of a linear function of time. The risk estimate (β) resulting from these analyses reflected the relative difference in slopes between the groups over 5 years of follow-up. To test for a difference that is not progressive but stable over time, a model without interaction term was fitted; the overall effect of the risk factor then reflected a constant effect in time. This model does not exclude patients in the case of missing radiographs and can deal with missing data, provided that it is at random (Knevel et al., 2010, manuscript under review). Patients with complete data sets are weighted more heavily in the analysis than patients with missing radiographs.
All factors that were associated with the progression of joint destruction were entered in a multivariate analysis to determine the variance of joint destruction explained by these factors. This variance was defined by comparing the residual variance of the analysis including all risk factors with the residual variance of the analysis including only the adjustment factor for treatment strategy (inclusion period). The proportional reduction of the residual variance was the explained variance of the risk factors analysed.
P < 0.05 were considered to be statistically significant. Since the aim of this study was to review baseline characteristics in relation to disease outcome, P-values were presented without corrections for multiple testing. Analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA).
A total of 177 (31%) UA patients developed RA. An overview of baseline characteristics associating with RA development and arthritis persistency is presented in Table 1. Some of the variables predictive for the development of RA were described previously [9–11]. Identified variables associating with the development of RA were patient characteristics (age, gender, having a positive family history of RA), morning stiffness, inflammatory characteristics (CRP, ESR and number of swollen joints), localization of involved joints and presence of autoantibodies [RF, anti-CCP2 and anti-modified citrullinated vimentin antibodies (anti-MCVs)]. The environmental factors such as smoking and BMI were not associated with progression from UA to RA. The acuteness of the start of the complaints was associated with RA development; UA patients with a gradual onset of symptoms had a 1.5 higher odds ratio (OR) of developing RA than patients with a subacute symptom onset. A longer duration of symptoms at first presentation was also associated with a higher risk of the development of RA.
Baseline characteristics of patients with UA in relation to the outcome measures: RA development and arthritis persistency
As the outcome measure of fulfilling the 1987 ACR criteria for RA might be subject to discussion (because these criteria were not designed to identify RA in an early phase) and to circular reasoning (because the presence of hand erosions are part of the ACR criteria), we also tested these baseline characteristics in relation to arthritis persistency, defined as the absence of sustained remission. During the 5-year period of follow-up, 210 UA patients achieved remission (39%). The median disease duration till remission achieved was 17 months [interquartile range (IQR) 6.3–37 months]. Factors significantly associated with disease persistency were inflammatory markers (the number of swollen joints, CRP and ESR) and presence of autoantibodies. Other characteristics such as the distribution of involved joints, the acuteness of the onset of the complaints and morning stiffness were not predictive of having a persistent form of arthritis.
Baseline characteristics of RA patients associated with the severity of joint destruction over time are presented in Table 2. The strongest association with the rate of joint destruction was seen for the presence of anti-CCP2. Anti-CCP-positive RA patients had a 2.4-times higher progression rate than anti-CCP-negative patients over the 5-year period. A similar effect was seen for the presence of IgM-RF. Higher levels of acute-phase reactants at first presentation were also associated with more severe joint damage over time. RA patients in whom initial joint symptoms were located at the lower extremities had a higher rate of joint destruction. Interestingly, the severity of morning stiffness at first presentation was not associated with the severity of joint destruction over time. The BMI was inversely correlated with the progression of joint destruction over time. Few genetic factors are convincingly reported to associate with progression of joint destruction. Here, we studied the HLA-DRB1 shared epitope alleles (HLA-SEs) alleles and CD40; both are identified risk factors for anti-CCP-positive RA only [10, 12]. Although presence of the HLA-SE alleles associated with the progression of joint destruction in RA, CD40did not reveal such an association in a cohort consisting of both anti-CCP-positive and anti-CCP-negative RA patients. All the analyses on the rate of joint destruction were adjusted for the treatment strategy applied; this variable was significantly associated with the rate of joint destruction in all performed analyses.
Baseline characteristics of patients with RA in relation to the outcome measures: rate of joint destruction and RA persistency
Since it is unclear whether the processes driving joint destruction are the same as those that drive RA persistency, predictive factors for both outcomes of RA were compared. Since the proportion of patients that achieved a sustained DMARD-free remission was 0.157, 84.3% of the patients were classified as having persistent RA. The median disease duration till remission was 40 months (IQR 25.5–66.5). The factors that were clearly associated with RA persistency were presence of autoantibodies, the HLA-SE alleles and the duration of symptoms at the first visit. A high BMI was associated with a higher chance of RA persistency. Although the characteristics indicative of the level of inflammation (CRP, ESR and SJC) were associated with severity of joint destruction, they were not predictive for having a persistent form of RA.
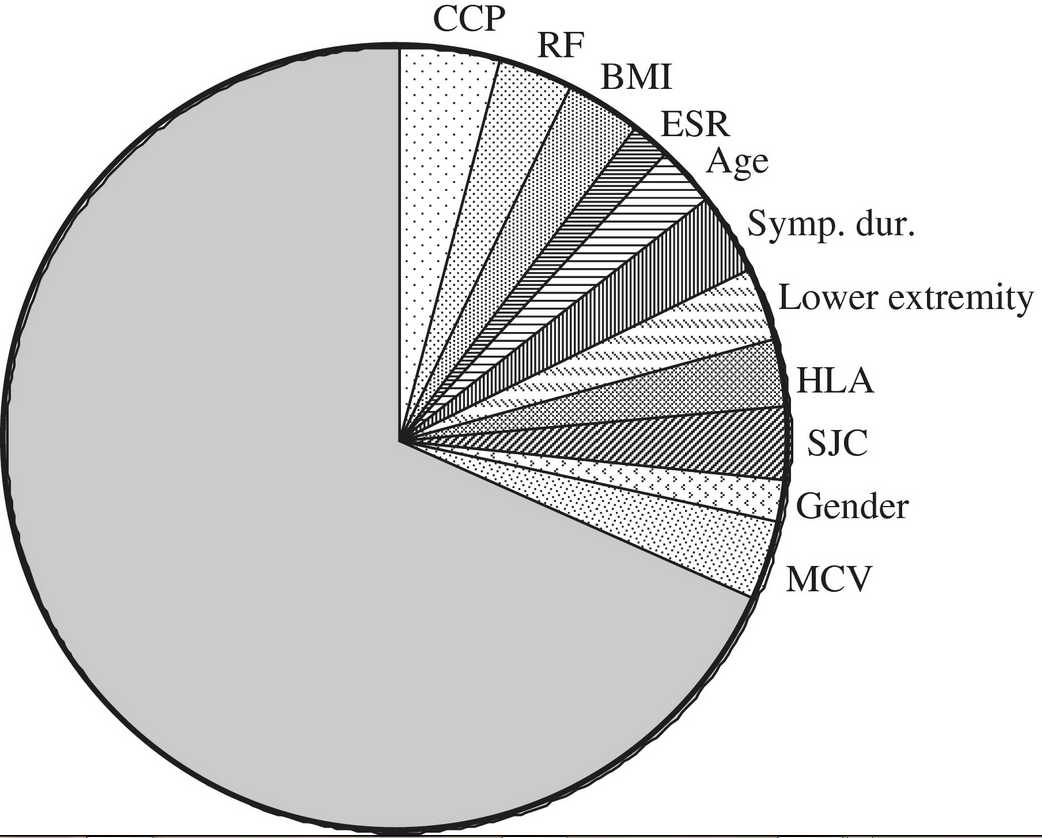
The total variance of joint destruction at 5 years explained by the baseline characteristics studied was 32%. Subsequently, we aimed to study the contribution of the individual risk factors to the explained variance. This was accomplished by calculating the proportion of the effect size of the individual factors in the multivariate analysis to the total effect. The proportional effect size of these variables is depicted in Fig. 1.
Contribution of baseline variables to the explained variance of SHS over 5 years. Presented is the explained variance at 5 years of baseline variables that were associated with the progression of joint destruction. Sympt. dur.: symptom duration at first visit; lower extremity: initial complaints at lower extremities vs upper extremities. All continuous variables were categorized in two groups in order to derive this figure; BMI was grouped in lower or higher than 25. Symptom duration at first visit was grouped in lower or higher than 12 weeks; SJC was grouped as fewer and more than 6 swollen joints; age under and above the median of 57 years; and ESR normal or elevated according to the reference value.
Accumulating evidence supports the relevance of initiating DMARD therapy as early as possible. Individualized treatment decision-making is hampered by the variability of the outcome of early arthritis. In the case of early UA, the question is when the DMARD therapy should be initiated. In early RA, it would be beneficial to recognize the patients who will have a severe disease course, since in these patients the benefits of early combination therapy with potent targeted therapies will outweigh the associated costs and risks of side effects. In this themed issue, risk factors for the outcome of UA and RA patients are explored based on the data of the Leiden EAC.
With regard to early UA, it was observed that predictive factors for the fulfilment of the 1987 ACR criteria for RA and for having persistent arthritis were largely similar. A predictive tool for RA development was derived before using a combination of identified risk factors [9]. This prediction rule is now well validated [13–15]. Since the present study did not intend to re-derive or improve this predictive tool, no multivariate regression analyses were performed in UA patients. Some studies tried to improve this prediction rule and assessed the additive value of baseline erosiveness and genetic markers [7, 16]. Unfortunately, these attempts did not result in an increased prognostic performance of this model. Further improvements of the model may be expected to come from ultrasound and MRI studies. Although at present not much data on ultrasound and MRI in unselected populations of UA patients are available, initial results are promising [17].
Fulfilling the 1987 ACR criteria as outcome of UA has the disadvantage that it may introduce some circular reasoning; in contrast, the difficulty with the outcome measure disease persistency is that classification depends on the duration of follow-up. In UA patients included in this study remission was achieved after a median period of 17 months, whereas in the RA patients the median disease duration till remission was 40 months. A too early comparison of disease outcomes may result in misclassification of potential remission patients into the persistent disease category. In order to diminish the risk of misclassification in this study, we chose to classify patients with ≥5years of arthritis as being persistent. This follow-up duration is arbitrary and results may have been slightly different in the case of a shorter or longer follow-up period being chosen.
The most potent predictors for having a persistent course of arthritis in UA patients and a persistent course of RA were the presence of autoantibodies. Inflammatory markers (the number of swollen joints, ESR and CRP) were associated with the development of RA and a persistent form of arthritis in UA patients as well as the severity of joint destruction in RA patients, which is in line with findings in older studies. However, no significant association between these inflammatory markers and disease persistency was found in RA patients [18, 19]. This may be due to the fact that the number of patients with sustained DMARD-free remission in RA was low, thereby reducing the power to identify significant associations with this outcome measure.
It is interesting to note that morning stiffness is strongly associated with the development of RA but not with disease persistency or the severity of joint destruction. Several explanations may account for this feature. One of them is that morning stiffness is mainly related to RA according to the 1987 criteria because of circular reasoning. Morning stiffness is not part of the 2010 EULAR/ACR criteria for RA and it would be an interesting subject for further studies to see whether the association between morning stiffness and the risk of RA is still present when the new definition of RA is used.
Other intriguing findings concern the observations on BMI. Obese RA patients are found to have less severe joint destruction. This observation was made not only in the present study but also in other populations [20–22]. The present study revealed that BMI was not associated with progression from UA to RA, but it was associated with having a persistent arthritis or persistent RA. Thus, this indicates that obese patients more often have persistent disease than non-obese arthritis patients. This observation is highly fascinating and may point to the notion that the role of fat tissue in RA is not completely clear. Fat tissue secretes pro-inflammatory as well as anti-inflammatory adipocytokines [23]. It is clear that some of the mechanisms of joint destruction like osteoclast activation are different from inflammatory pathways and as such it is tempting to speculate that diverse adipocytokines may have different preferential effects on arthritis persistency and on joint destruction.
The associations between disease outcomes and involvement of the joints of the lower or upper extremities were different for patients with UA and RA. Whereas within UA the presence of arthritis in lower extremities was associated with a lower OR for RA, within RA patients it was associated with a higher rate of joint destruction. This finding is in line with previous findings demonstrating that patients presenting with knee arthritis had a more severe rate of joint destruction compared with patients without knee arthritis, when measured using destruction of small feet and hand joints [24].
Emerging evidence indicates that anti-CCP-positive and anti-CCP-negative RA are subsets of RA with differences in the underlying pathological mechanisms [25, 26]. The present study addressed all UA patients and RA patients; stratified analyses on anti-CCP-positive and anti-CCP-negative patients were not performed. This may be an explanation why CD40, a genetic risk factor of joint destruction in anti-CCP-positive RA is not associated with the rate of joint destruction in the whole RA population [12].
The baseline characteristics associated with the severity of joint destruction in RA were mainly autoantibodies and other patient characteristics, and to a lesser extent factors expressing the level of inflammation. Although the present study did not evaluate the contribution of inflammation over time on the final level of joint destruction, such analyses have been performed before. Some of these studies also suggested that the largest part of joint destruction is not directly related to cumulative inflammatory markers [27].
The data presented are limited to data of the Leiden EAC cohort. However, many of the associating risk factors for UA and RA are observed in individual studies originating from different early arthritis cohorts as well [28–33].
The proportion of the explained variance in progression of joint destruction by the identified risk factors was 32%. Although no clear guidelines are available regarding what level of explained variance is required in order to derive a prediction model with an adequate discriminative performance, previous investigations and experience [9, 34] are highly suggestive that the explained variance is insufficient to proceed with a derivation of a prediction rule for the rate of joint destruction in RA. This notion is exemplified by recent attempts to derive prediction models; with the current prediction rules ∼50% of the RA patients could not be adequately classified [34–36].
In conclusion, although the processes determining the persistency and severity of arthritis are incompletely understood, the identification of risk factors may help in individualization of therapy in patients with recent-onset UA. In RA, in contrast, the currently known risk factors for a progressive destructive disease course explain only part of the individual differences in level of joint destruction and more risk factors need to be identified in order to achieve individualized treatment decision-making.

Funding: This work is supported by The Dutch Arthritis Foundation and by The Netherlands Organization for Health Research.
Disclosure statement: The authors have declared no conflicts of interest.
标签:
原文地址:http://www.cnblogs.com/biopy/p/4784881.html