标签:
http://www.cellimagelibrary.org/images/43705
word count
---------------------
caspase 31
caspases 22
membrane 16
tnf 12
proapoptotic 12
bcl 12
hela 10
fas 9
cdv 9
apaf 8
stimuli 7
greek 7
signals 6
orov 6
knockout 6
intracellular 6
cytochrome 6
caspaseindependent 6
tnfr 5
tnfalpha 5
signaling 5
nuclear 5
necrotic 5
necrosis 5
kerr 5
inhibitory 5
extrinsic 5
bax 5
treatments 4
transduction 4
subcellular 4
removal 4
phosphatidylserine 4
permeability 4
necroptotic 4
morphology 4
lymphoma 4
lymphocytes 4
intrinsic 4
hpv 4
fadd 4
degradation 4
cormack 4
canine 4
bak 4
wyllie 3
smac 3
proteases 3
protease 3
procaspase 3
phenomenon 3
oropouche 3
organelles 3
nfκb 3
mrna 3
morphological 3
microscopy 3
marrow 3
mac 3
knockouts 3
irradiation 3
inactive 3
iaps 3
homologs 3
fluorocytometry 3
finally 3
extracellular 3
excess 3
embryonic 3
dying 3
cytotoxic 3
currie 3
cleavage 3
characteristic 3
cascade 3
akt 3
aif 3
aberdeen 3
>>>
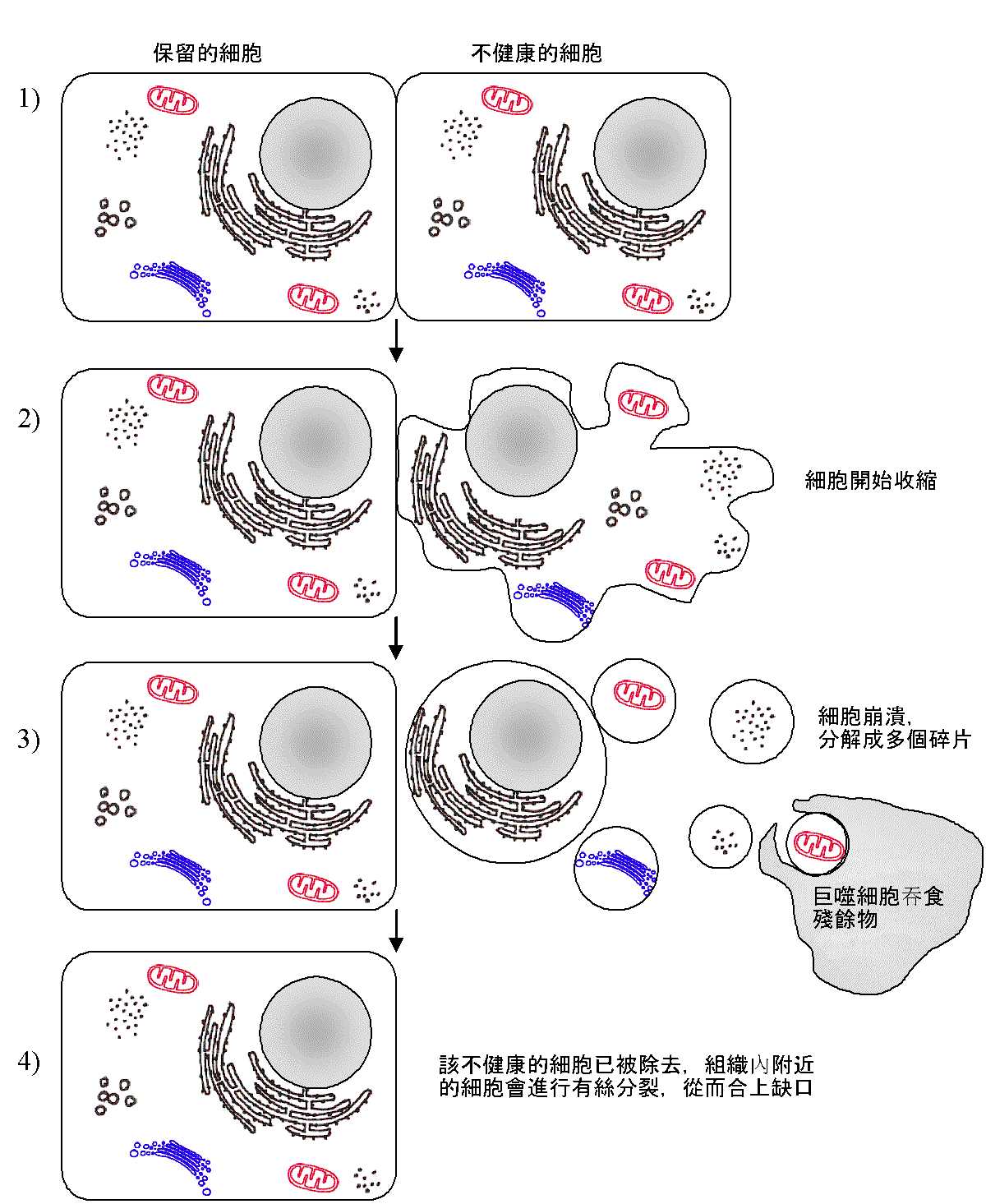
细胞凋亡(英语:apoptosis,源自希腊语:απ?πτωσις,有堕落,死亡之意),为一种细胞程序性死亡。相对于细胞坏死(necrosis),细胞凋亡是细胞主动实施的。细胞凋亡一般由生理或病理性因素引起。而细胞坏死则主要为缺氧造成,两者可以很容易通过观察区分开来。在细胞凋亡过程中,细胞缩小,DNA被核酸内切酶降解成180bp-200bp片段属于有层次之断裂,(可以通过凝胶电泳证明),而细胞坏死时,细胞肿胀,细胞膜被破坏,通透性改变。细胞器散落到细胞间质,需要巨噬细胞去清除,结果是该局部组织发炎。相比起细胞坏死,细胞凋亡是更常见的细胞死亡形式。
细胞凋亡是动物发育过程中的必经之路:
在人体胚胎发育中,四肢的发育也是一个很好的例子。胚胎长到第5周时出现扁盘状的肢体萌芽,手指和脚趾之间的蹼消失,四肢才能顺利形成其最终形态。
就是在成熟的个体中,细胞凋亡也是必不可少的:
目前在细胞凋亡与癌症发生和各种自体免疫性疾病之间的关系有着很多研究。人们希望激发癌细胞的细胞凋亡,以达到消弭癌症的目的。另有为了维持人类大脑的体积,使细胞凋亡的基因在人类身上受到了抑制的说法[1][2]。
细胞凋亡在神经退化性疾病中所扮演的角色(如阿兹海默氏症(失智症的类型之一)、亨丁顿舞蹈症(Huntington‘s disease)、帕金森病(Parkinson‘s Disease)、肌肉萎缩性侧索硬化症(ALS),是目前热门的研究话题,人们在这个领域也进行着很多研究。
细胞凋亡过程可分为两阶段:开始阶段和效应阶段。在开始阶段又可分为两个途径:外始式和内始式。
外始式途径:
外始式途径是通过TNF受体家族(如CD95)的受体与配体结合开始的。这些配体有癌症坏死因子(TNF),和其他细胞因子,后者可以由如T淋巴细胞分泌。 在FADD(Fas偶联死亡区域蛋白Fas-associated death domain protein)的协助下,受体不断在细胞质中收集半胱天冬酶原8(Procaspase 8)。后者通过高密度自催化激活自身。活化的半胱天冬酶8(Caspase 8)将会引发所谓的Caspase级联反应。
因此在艾滋病人体内,很多未受感染的白血球也会凋亡:HIV病毒通过Nef蛋白质激发未受感染的防御细胞的程序性死亡。抑制剂盐酸法舒地尔可以阻断这一机制。
内始式途径:
内始式途径始于肿瘤抑制基因如p53,一个转录因子,它会受DNA损伤激发。P53能刺激Bcl-2家族中于细胞凋亡前起作用的成员(如Bax, Bad)的表达。这将导致线粒体内外膜间的物质释放,如细胞色素C和Smac/DIABLO,它们都是作用于细胞凋亡前的物质。细胞色素C和胞质中的Apaf-1以及Procaspase 9共同组成所谓的凋亡体,其实就是Caspase 9的活化形式。它和Caspase 8一样引起Caspase级联反应。
Caspase级联反应和效应caspase:
所谓的效应caspase,指的是Caspase 3, 6和7这些能引起细胞的程序性死亡的蛋白酶。一方面它们通过有限的蛋白质水解酶激活下游的目标蛋白(如Caspase激活的去氧核糖核酸酶, CAD,或是其他的Caspase)。另一方面它们参与核纤肽(在细胞膜上)和肌动蛋白(细胞骨架的成分)的分解过程。 另一方面,DNA的修复会为caspase介导的阻抗反应而受到抑制。
最后细胞慢慢地缩小为一小颗粒,并且为邻近具有吞噬能力的细胞所吞噬(胞葬作用)。相对于细胞坏死,细胞膜在凋亡过程中保持完整。
细胞色素C从线粒体中溢出到细胞质,这是细胞凋亡的标志之一。这在外始式途径中是在凋亡过程后期才出现的,这里它反而是凋亡的结果,而不再是在内始式途径中所担任的激发者的角色了。
在外始式途径中,又分为主动(由受体的激活开始)和被动(由生长因子,如神经营养素的排出引起)两种形式。
最重要的抑制细胞凋亡蛋白质有:Bcl-2家族中的抗细胞凋亡成员(Bcl-2和Bcl-xL),蛋白激酶B和Trk受体家族(参看神经营养素)还有IAP蛋白(inhibitor-of-apoptosis protein)
Apoptosis (/?æp??to?s?s/;[2][3] from Ancient Greek ?π? apo, "by, from, of, since, than" and πτ?σις ptōsis, "fall") is the process of programmed cell death (PCD) that may occur in multicellular organisms.[4]Biochemical events lead to characteristic cell changes (morphology) and death. These changes include blebbing, cell shrinkage,nuclear fragmentation, chromatin condensation, chromosomal DNA fragmentation, and global mRNA decay.
In contrast to necrosis, which is a form of traumatic cell death that results from acute cellular injury, apoptosis is a highly regulated and controlled process that confers advantages during an organism‘s lifecycle. For example, the separation of fingers and toes in a developing humanembryo occurs because cells between the digits undergo apoptosis. Unlike necrosis, apoptosis produces cell fragments called apoptotic bodies that phagocytic cells are able to engulf and quickly remove before the contents of the cell can spill out onto surrounding cells and cause damage.[5]
Between 50 and 70 billion cells die each day due to apoptosis in the average human adult. For an average child between the ages of 8 and 14, approximately 20 billion to 30 billion cells die a day.[6]
Research in and around apoptosis has increased substantially since the early 1990s. In addition to its importance as a biological phenomenon, defective apoptotic processes have been implicated in a wide variety of diseases. Excessive apoptosis causes atrophy, whereas an insufficient amount results in uncontrolled cell proliferation, such as cancer. Some factors like fas, caspases (C-cysteine rich, asp- aspartic acid moiety containing, ase – proteases) etc. promote apoptosis, while members ofBcl-2 inhibit apoptosis.
German scientist Karl Vogt was first to describe the principle of apoptosis in 1842. In 1885, anatomist Walther Flemming delivered a more precise description of the process of programmed cell death. However, it was not until 1965 that the topic was resurrected. While studying tissues using electron microscopy, John Foxton Ross Kerr at University of Queensland was able to distinguish apoptosis from traumatic cell death.[7] Following the publication of a paper describing the phenomenon, Kerr was invited to join Alastair R Currie, as well as Andrew Wyllie, who was Currie‘s graduate student,[8] at University of Aberdeen. In 1972, the trio published a seminal article in the British Journal of Cancer.[9]Kerr had initially used the term programmed cell necrosis, but in the article, the process of natural cell death was called apoptosis. Kerr, Wyllie and Currie credited James Cormack, a professor of Greek language at University of Aberdeen, with suggesting the term apoptosis. Kerr received the Paul Ehrlich and Ludwig Darmstaedter Prize on March 14, 2000, for his description of apoptosis. He shared the prize with Boston biologist H. Robert Horvitz.[10]
For many years, the terms "apoptosis" and "programmed cell death" were not highly cited. What transformed cell death from obscurity to a major field of research were two things: the identification of components of the cell death control and effector mechanisms, and the linkage of abnormalities in cell death to human disease, in particular cancer.
The 2002 Nobel Prize in Medicine was awarded to Sydney Brenner, Horvitz and John E. Sulstonfor their work identifying genes that control apoptosis. The genes were identified by studies in the nematode C. elegans and these same genes function in humans for apoptosis.

In Greek, apoptosis translates to the "dropping off" of petals or leaves from plants or trees. Cormack, professor of Greek language, reintroduced the term for medical use as it had a medical meaning for the Greeks over two thousand years before.Hippocrates used the term to mean "the falling off of the bones". Galen extended its meaning to "the dropping of the scabs". Cormack was no doubt aware of this usage when he suggested the name. Debate continues over the correct pronunciation, with opinion divided between a pronunciation with the second p silent (/æp??to?s?s/ ap-?-toh-sis[2][11]) and the second p pronounced (/æp?p?to?s?s/),[2][12] as in the original Greek.[citation needed] In English, the p of the Greek -pt- consonant cluster is typically silent at the beginning of a word (e.g. pterodactyl, Ptolemy), but articulated when used in combining forms preceded by a vowel, as in helicopter or the orders of insects: diptera, lepidoptera, etc.
In the original Kerr Wyllie and Currie paper, British Journal of Cancer, 1972 Aug;26(4):239-57, there is a footnote regarding the pronunciation:
"We are most grateful to Professor James Cormack of the Department of Greek, University of Aberdeen, for suggesting this term. The word "apoptosis" (?π?πτωσις) is used in Greek to describe the "dropping off" or "falling off" of petals from flowers, or leaves from trees. To show the derivation clearly, we propose that the stress should be on the penultimate syllable, the second half of the word being pronounced like "ptosis" (with the "p" silent), which comes from the same root "to fall", and is already used to describe the drooping of the upper eyelid."
A cell initiates intracellular apoptotic signaling in response to a stress, which may bring about cell suicide. The binding of nuclear receptors byglucocorticoids,[13] heat,[13] radiation,[13] nutrient deprivation,[13] viral infection,[13] hypoxia[13] and increased intracellular calcium concentration,[14] for example, by damage to the membrane, can all trigger the release of intracellular apoptotic signals by a damaged cell. A number of cellular components, such as poly ADP ribose polymerase, may also help regulate apoptosis.[15]
Before the actual process of cell death is precipitated by enzymes, apoptotic signals must cause regulatory proteins to initiate the apoptosis pathway. This step allows apoptotic signals to cause cell death, or the process to be stopped, should the cell no longer need to die. Several proteins are involved, but two main methods of regulation have been identified: targeting mitochondriafunctionality, or directly transducing the signal viaadaptor proteins to the apoptotic mechanisms. Another extrinsic pathway for initiation identified in several toxin studies is an increase in calcium concentration within a cell caused by drug activity, which also can cause apoptosis via a calcium binding protease calpain.
The mitochondria are essential to multicellular life. Without them, a cell ceases to respire aerobically and quickly dies. This fact forms the basis for some apoptotic pathways. Apoptotic proteins that target mitochondria affect them in different ways. They may cause mitochondrial swelling through the formation of membrane pores, or they may increase the permeability of the mitochondrial membrane and cause apoptotic effectors to leak out.[13] These are very closely related to intrinsic pathway, and tumors arise more frequently through intrinsic pathway than the extrinsic pathway because of sensitivity.[16] There is also a growing body of evidence indicating that nitric oxide is able to induce apoptosis by helping to dissipate the membrane potential of mitochondria and therefore make it more permeable.[17] Nitric oxide has been implicated in initiating and inhibiting apoptosis through its possible action as a signal molecule of subsequent pathways that activate apoptosis.[18][citation needed]
Mitochondrial proteins known as SMACs (second mitochondria-derived activator of caspases) are released into the cytosol following an increase in permeability. SMAC binds toinhibitor of apoptosis proteins (IAPs) and deactivates them, preventing the IAPs from arresting the apoptotic process and therefore allowing apoptosis to proceed. IAP also normally suppresses the activity of a group of cysteine proteases called caspases,[19] which carry out the degradation of the cell, therefore the actual degradation enzymes can be seen to be indirectly regulated by mitochondrial permeability.
Cytochrome c is also released from mitochondria due to formation of a channel, themitochondrial apoptosis-induced channel (MAC), in the outer mitochondrial membrane,[20] and serves a regulatory function as it precedes morphological change associated with apoptosis.[13] Once cytochrome c is released it binds with Apoptotic protease activating factor – 1 (Apaf-1) and ATP, which then bind to pro-caspase-9 to create a protein complex known as an apoptosome. The apoptosome cleaves the pro-caspase to its active form ofcaspase-9, which in turn activates the effector caspase-3.
MAC (not to be confused with the Membrane Attack Complex formed by complement activation, also commonly denoted as MAC), also called "Mitochondrial Outer Membrane Permeabilization Pore" is regulated by various proteins, such as those encoded by the mammalian Bcl-2 familyof anti-apoptopic genes, the homologs of the ced-9 gene found in C. elegans.[21][22] Bcl-2proteins are able to promote or inhibit apoptosis by direct action on MAC/MOMPP. Bax and/or Bak form the pore, while Bcl-2, Bcl-xL or Mcl-1 inhibit its formation.
Two theories of the direct initiation of apoptotic mechanisms in mammals have been suggested: the TNF-induced(tumour necrosis factor) model and the Fas-Fasligand-mediatedmodel, both involving receptors of theTNF receptor(TNFR) family[23]coupled to extrinsic signals.
TNF-alpha is acytokineproduced mainly by activatedmacrophages, and is the major extrinsic mediator of apoptosis. Most cells in the human body have two receptors for TNF-alpha: TNFR1and TNFR2. The binding of TNF-alpha to TNFR1 has been shown to initiate the pathway that leads to caspase activation via the intermediate membrane proteins TNF receptor-associated death domain (TRADD) and Fas-associated death domain protein (FADD). cIAP1/2 can inhibit TNF-α signaling by binding to TRAF2. FLIP inhibits the activation of caspase-8.[24] Binding of this receptor can also indirectly lead to the activation of transcription factorsinvolved in cell survival and inflammatory responses.[25] However, signalling through TNFR1 might also induce apoptosis in a caspase-independent manner.[26] The link between TNF-alpha and apoptosis shows why an abnormal production of TNF-alpha plays a fundamental role in several human diseases, especially in autoimmune diseases.
The fas receptor First apoptosis signal (fas) – (also known as Apo-1 or CD95) binds theFas ligand (FasL), a transmembrane protein part of the TNF family.[23] The interaction between Fas and FasL results in the formation of the death-inducing signaling complex(DISC), which contains the FADD, caspase-8 and caspase-10. In some types of cells (type I), processed caspase-8 directly activates other members of the caspase family, and triggers the execution of apoptosis of the cell. In other types of cells (type II), the Fas-DISC starts a feedback loop that spirals into increasing release of proapoptotic factors from mitochondria and the amplified activation of caspase-8.[27]
Following TNF-R1 and Fas activation in mammalian cells a balance between proapoptotic (BAX,[28] BID, BAK, or BAD) and anti-apoptotic (Bcl-Xl and Bcl-2) members of the Bcl-2family is established. This balance is the proportion of proapoptotic homodimers that form in the outer-membrane of the mitochondrion. The proapoptotic homodimers are required to make the mitochondrial membrane permeable for the release of caspase activators such as cytochrome c and SMAC. Control of proapoptotic proteins under normal cell conditions of nonapoptotic cells is incompletely understood, but in general, Bax or Bak are activated by the activation of BH3-only proteins, part of the Bcl-2 family.
Caspases play the central role in the transduction of DR apoptotic signals. Caspases are proteins that are highly conserved, cysteine-dependent aspartate-specific proteases. There are two types of caspases: initiator caspases, caspase 2,8,9,10,11,12, and effector caspases, caspase 3,6,7. The activation of initiator caspases requires binding to specific oligomeric activator protein. Effector caspases are then activated by these active initiator caspases through proteolytic cleavage. The active effector caspases then proteolytically degrade a host of intracellular proteins to carry out the cell death program.
There also exists a caspase-independent apoptotic pathway that is mediated by AIF (apoptosis-inducing factor).[29]
Many pathways and signals lead to apoptosis, but there is only one mechanism that actually causes the death of a cell.[citation needed] After a cell receives stimulus, it undergoes organized degradation of cellular organelles by activated proteolytic caspases. In addition to the destruction of cellular organelles, mRNA is rapidly and globally degraded by a mechanism that is not yet fully characterized.[30] mRNA decay is triggered very early in apoptosis. A cell undergoing apoptosis shows a characteristic morphology:
Apoptosis progresses quickly and its products are quickly removed, making it difficult to detect or visualize. During karyorrhexis, endonuclease activation leaves short DNA fragments, regularly spaced in size. These give a characteristic "laddered" appearance onagar gel after electrophoresis. Tests for DNA laddering differentiate apoptosis fromischemic or toxic cell death.[35]
The removal of dead cells by neighboring phagocytic cells has been termed efferocytosis.[36]Dying cells that undergo the final stages of apoptosis display phagocytotic molecules, such as phosphatidylserine, on their cell surface.[37] Phosphatidylserine is normally found on the inner leaflet surface of the plasma membrane, but is redistributed during apoptosis to the extracellular surface by a protein known as scramblase.[38] These molecules mark the cell for phagocytosis by cells possessing the appropriate receptors, such as macrophages.[39] Upon recognition, the phagocyte reorganizes its cytoskeleton for engulfment of the cell. The removal of dying cells by phagocytes occurs in an orderly manner without eliciting an inflammatory response.[39]
Many knock-outs have been made in the apoptosis pathways to test the function of each of the proteins. Several caspases, in addition to APAF-1 and FADD, have been mutated to determine the new phenotype. In order to create a tumor necrosis factor (TNF) knockout, an exon containing the nucleotides 3704-5364 was removed from the gene. This exon encodes a portion of the mature TNF domain, as well as the leader sequence, which is a highly conserved region necessary for proper intracellular processing. TNF-/- mice develop normally and have no gross structural or morphological abnormalities. However, upon immunization with SRBC (sheep red blood cells), these mice demonstrated a deficiency in the maturation of an antibody response; they were able to generate normal levels of IgM, but could not develop specific IgG levels. Apaf-1 is the protein that turns on caspase 9 by cleavage to begin the caspase cascade that leads to apoptosis. Since a -/- mutation in the APAF-1 gene is embryonic lethal, a gene trap strategy was used in order to generate an APAF-1 -/- mouse. This assay is used to disrupt gene function by creating an intragenic gene fusion. When an APAF-1 gene trap is introduced into cells, many morphological changes occur, such as spina bifida, the persistence of interdigital webs, and open brain. In addition, after embryonic day 12.5, the brain of the embryos showed several structural changes. APAF-1 cells are protected from apoptosis stimuli such as irradiation. A BAX-1 knock-out mouse exhibits normal forebrain formation and a decreased programmed cell death in some neuronal populations and in the spinal cord, leading to an increase in motor neurons.
The caspase proteins are integral parts of the apoptosis pathway, so it follows that knock-outs made have varying damaging results. A caspase 9 knock-out leads to a severe brain malformation. A caspase 8 knock-out leads to cardiac failure and thus embryonic lethality. However, with the use of cre-lox technology, a caspase 8 knock-out has been created that exhibits an increase in peripheral T cells, an impaired T cell response, and a defect in neural tube closure. These mice were found to be resistant to apoptosis mediated by CD95, TNFR, etc. but not resistant to apoptosis caused by UV irradiation, chemotherapeutic drugs, and other stimuli. Finally, a caspase 3 knock-out was characterized by ectopic cell masses in the brain and abnormal apoptotic features such as membrane blebbing or nuclear fragmentation. A remarkable feature of these KO mice is that they have a very restricted phenotype: Casp3, 9, APAF-1 KO mice have deformations of neural tissue and FADD and Casp 8 KO showed defective heart development, however in both types of KO other organs developed normally and some cell types were still sensitive to apoptotic stimuli suggesting that unknown proapoptotic pathways exist.
In order to perform analysis of apoptotic versus necrotic (necroptotic) cells, one can do analysis of morphology by time-lapse microscopy, flow fluorocytometry, and transmission electron microscopy. There are also various biochemical techniques for analysis of cell surface markers (phosphatidylserine exposure versus cell permeability by flow fluorocytometry), cellular markers such as DNA fragmentation (flow fluorocytometry), caspase activation, Bid cleavage, and cytochrome c release (Western blotting). It is important to know how primary and secondary necrotic cells can be distinguished by analysis of supernatant for caspases, HMGB1, and release of cytokeratin 18. However, no distinct surface or biochemical markers of necrotic cell death have been identified yet, and only negative markers are available. These include absence of apoptotic parameters (caspase activation, cytochrome c release, and oligonucleosomal DNA fragmentation) and differential kinetics of cell death markers (phosphatidylserine exposure and cell membrane permeabilization). A selection of techniques that can be used to distinguish apoptosis from necroptotic cells could be found in these references.[40][40][40]

The many different types of apoptotic pathways contain a multitude of different biochemical components, many of them not yet understood.[41] As a pathway is more or less sequential in nature, it is a victim of causality; removing or modifying one component leads to an effect in another. In a living organism, this can have disastrous effects, often in the form of disease or disorder. A discussion of every disease caused by modification of the various apoptotic pathways would be impractical, but the concept overlying each one is the same: The normal functioning of the pathway has been disrupted in such a way as to impair the ability of the cell to undergo normal apoptosis. This results in a cell that lives past its "use-by-date" and is able to replicate and pass on any faulty machinery to its progeny, increasing the likelihood of the cell‘s becoming cancerous or diseased.
A recently described example of this concept in action can be seen in the development of a lung cancer called NCI-H460.[42] The X-linked inhibitor of apoptosis protein (XIAP) is overexpressed in cells of the H460cell line. XIAPs bind to the processed form of caspase-9, and suppress the activity of apoptotic activatorcytochrome c, therefore overexpression leads to a decrease in the amount of proapoptotic agonists. As a consequence, the balance of anti-apoptotic and proapoptotic effectors is upset in favour of the former, and the damaged cells continue to replicate despite being directed to die.
The tumor-suppressor protein p53 accumulates when DNA is damaged due to a chain of biochemical factors. Part of this pathway includes alpha-interferon and beta-interferon, which induce transcription of the p53 gene, resulting in the increase of p53 protein level and enhancement of cancer cell-apoptosis.[43] p53 prevents the cell from replicating by stopping the cell cycle at G1, or interphase, to give the cell time to repair, however it will induce apoptosis if damage is extensive and repair efforts fail. Any disruption to the regulation of the p53 or interferon genes will result in impaired apoptosis and the possible formation of tumors.
Inhibition of apoptosis can result in a number of cancers, autoimmune diseases, inflammatory diseases, and viral infections. It was originally believed that the associated accumulation of cells was due to an increase in cellular proliferation, but it is now known that it is also due to a decrease in cell death. The most common of these diseases is cancer, the disease of excessive cellular proliferation, which is often characterized by an overexpression of IAP family members. As a result, the malignant cells experience an abnormal response to apoptosis induction: Cycle-regulating genes (such as p53, ras or c-myc) are mutated or inactivated in diseased cells, and further genes (such as bcl-2) also modify their expression in tumors.
Apoptosis in HeLa cells is inhibited by proteins produced by the cell; these inhibitory proteins target retinoblastoma tumor-suppressing proteins.[44] These tumor-suppressing proteins regulate the cell cycle, but are rendered inactive when bound to an inhibitory protein.[44] HPV E6 and E7 are inhibitory proteins expressed by the human papillomavirus, HPV being responsible for the formation of the cervical tumor from which HeLa cells are derived.[45] HPV E6 causes p53, which regulates the cell cycle, to become inactive.[46] HPV E7 binds to retinoblastoma tumor suppressing proteins and limits its ability to control cell division.[46] These two inhibitory proteins are partially responsible for HeLa cells‘ immortality by inhibiting apoptosis to occur.[47] CDV (Canine Distemper Virus) is able to induce apoptosis despite the presence of these inhibitory proteins. This is an importantoncolytic property of CDV: this virus is capable of killing canine lymphoma cells. Oncoproteins E6 and E7 still leave p53 inactive, but they are not able to avoid the activation of caspases induced from the stress of viral infection. These oncolytic properties provided a promising link between CDV and lymphoma apoptosis, which can lead to development of alternative treatment methods for both canine lymphoma and human non-Hodgkin lymphoma. Defects in the cell cycle are thought to be responsible for the resistance to chemotherapy or radiation by certain tumor cells, so a virus that can induce apoptosis despite defects in the cell cycle is useful for cancer treatment.[47]
The main method of treatment for death signaling-related diseases involves either increasing or decreasing the susceptibility of apoptosis in diseased cells, depending on whether the disease is caused by either the inhibition of or excess apoptosis. For instance, treatments aim to restore apoptosis to treat diseases with deficient cell death, and to increase the apoptotic threshold to treat diseases involved with excessive cell death. To stimulate apoptosis, one can increase the number of death receptor ligands (such as TNF or TRAIL), antagonize the anti-apoptotic Bcl-2 pathway, or introduce Smac mimetics to inhibit the inhibitor (IAPs). The addition of agents such as Herceptin, Iressa, or Gleevec works to stop cells from cycling and causes apoptosis activation by blocking growth and survival signaling further upstream. Finally, adding p53-MDM2 complexes displaces p53 and activates the p53 pathway, leading to cell cycle arrest and apoptosis. Many different methods can be used either to stimulate or to inhibit apoptosis in various places along the death signaling pathway.[48]
Apoptosis is a multi-step, multi-pathway cell-death programme that is inherent in every cell of the body. In cancer, the apoptosis cell-division ratio is altered. Cancer treatment by chemotherapy and irradiation kills target cells primarily by inducing apoptosis.
On the other hand, loss of control of cell death (resulting in excess apoptosis) can lead to neurodegenerative diseases, hematologic diseases, and tissue damage. The progression of HIV is directly linked to excess, unregulated apoptosis. In a healthy individual, the number of CD4+ lymphocytes is in balance with the cells generated by the bone marrow; however, in HIV-positive patients, this balance is lost due to an inability of the bone marrow to regenerate CD4+ cells. In the case of HIV, CD4+ lymphocytes die at an accelerated rate through uncontrolled apoptosis, when stimulated.
Treatments aiming to inhibit works to block specific caspases. Finally, the Akt protein kinase promotes cell survival through two pathways. Akt phosphorylates and inhibits Bas (a Bcl-2 family member), causing Bas to interact with the 14-3-3 scaffold, resulting in Bcl dissociation and thus cell survival. Akt also activates IKKα, which leads to NF-κB activation and cell survival. Active NF-κB induces the expression of anti-apoptotic genes such as Bcl-2, resulting in inhibition of apoptosis. NF-κB has been found to play both an antiapoptotic role and a proapoptotic role depending on the stimuli utilized and the cell type.[49]
The progression of the human immunodeficiency virus infection into AIDS is due primarily to the depletion of CD4+ T-helper lymphocytes in a manner that is too rapid for the body‘s bone marrow to replenish the cells, leading to a compromised immune system. One of the mechanisms by which T-helper cells are depleted is apoptosis, which results from a series of biochemical pathways:[50]
Cells may also die as direct consequences of viral infections. HIV-1 expression induces tubular cell G2/M arrest and apoptosis.[51] The progression from HIV to AIDS is not immediate or even necessarily rapid; HIV‘s cytotoxic activity toward CD4+ lymphocytes is classified as AIDS once a given patient‘s CD4+ cell count falls below 200.[52]
Viral induction of apoptosis occurs when one or several cells of a living organism are infected with a virus, leading to cell death. Cell death in organisms is necessary for the normal development of cells and the cell cycle maturation.[53] It is also important in maintaining the regular functions and activities of cells.
Viruses can trigger apoptosis of infected cells via a range of mechanisms including:
Canine distemper virus (CDV) is known to cause apoptosis in central nervous system and lymphoid tissue of infected dogs in vivo and in vitro.[55] Apoptosis caused by CDV is typically induced via the extrinsic pathway, which activates caspases that disrupt cellular function and eventually leads to the cells death.[44] In normal cells, CDV activates caspase-8 first, which works as the initiator protein followed by the executioner protein caspase-3.[44] However, apoptosis induced by CDV in HeLa cells does not involve the initiator protein caspase-8. HeLa cell apoptosis caused by CDV follows a different mechanism than that in vero cell lines.[44] This change in the caspase cascade suggests CDV induces apoptosis via the intrinsic pathway, excluding the need for the initiator caspase-8. The executioner protein is instead activated by the internal stimuli caused by viral infection not a caspase cascade.[44]
The Oropouche virus (OROV) is found in the family Bunyaviridae. The study of apoptosis brought on by Bunyaviridae was initiated in 1996, when it was observed that apoptosis was induced by the La Crosse virus into the kidney cells of baby hamsters and into the brains of baby mice.[56]
OROV is a disease that is transmitted between humans by the biting midge (Culicoides paraensis).[57] It is referred to as a zoonotic arbovirus and causes febrile illness, characterized by the onset of a sudden fever known as Oropouche fever.[58]
The Oropouche virus also causes disruption in cultured cells – cells that are cultivated in distinct and specific conditions. An example of this can be seen in HeLa cells, whereby the cells begin to degenerate shortly after they are infected.[56]
With the use of gel electrophoresis, it can be observed that OROV causes DNA fragmentation in HeLa cells. It can be interpreted by counting, measuring, and analyzing the cells of the Sub/G1 cell population.[56] When HeLA cells are infected with OROV, the cytochrome C is released from the membrane of the mitochondria, into the cytosol of the cells. This type of interaction shows that apoptosis is activated via an intrinsic pathway.[53]
In order for apoptosis to occur within OROV, viral uncoating, viral internalization, along with the replication of cells is necessary. Apoptosis in some viruses is activated by extracellular stimuli. However, studies have demonstrated that the OROV infection causes apoptosis to be activated through intracellular stimuli and involves the mitochondria.[56]
Many viruses encode proteins that can inhibit apoptosis.[59] Several viruses encode viral homologs of Bcl-2. These homologs can inhibit proapoptotic proteins such as BAX and BAK, which are essential for the activation of apoptosis. Examples of viral Bcl-2 proteins include the Epstein-Barr virus BHRF1 protein and the adenovirus E1B 19K protein.[60] Some viruses express caspase inhibitors that inhibit caspase activity and an example is the CrmA protein of cowpox viruses. Whilst a number of viruses can block the effects of TNF and Fas. For example, the M-T2 protein of myxoma viruses can bind TNF preventing it from binding the TNF receptor and inducing a response.[61] Furthermore, many viruses express p53 inhibitors that can bind p53 and inhibit its transcriptional transactivation activity. As a consequence, p53 cannot induce apoptosis, since it cannot induce the expression of proapoptotic proteins. The adenovirus E1B-55K protein and the hepatitis B virus HBx protein are examples of viral proteins that can perform such a function.[62]
Viruses can remain intact from apoptosis in particular in the latter stages of infection. They can be exported in the apoptotic bodies that pinch off from the surface of the dying cell, and the fact that they are engulfed by phagocytes prevents the initiation of a host response. This favours the spread of the virus.[61]
Programmed cell death in plants has a number of molecular similarities to that of animal apoptosis, but it also has differences, notable ones being the presence of a cell wall and the lack of an immune system that removes the pieces of the dead cell. Instead of an immune response, the dying cell synthesizes substances to break itself down and places them in avacuole that ruptures as the cell dies. Whether this whole process resembles animal apoptosis closely enough to warrant using the name apoptosis (as opposed to the more general programmed cell death) is unclear.[63]
The characterization of the caspases allowed the development of caspase inhibitors, which can be used to determine whether a cellular process involves active caspases. Using these inhibitors it was discovered that cells can die while displaying a morphology similar to apoptosis without caspase activation.[64] Later studies linked this phenomenon to the release of AIF (apoptosis-inducing factor) from the mitochondria and its translocation into the nucleus mediated by its NLS (nuclear localization signal). Inside the mitochondria, AIF is anchored to the inner membrane. In order to be released, the protein is cleaved by a calcium-dependent calpain protease.
In 2003, a method was developed for predicting subcellular location of apoptosis proteins.[65] Subsequent to this, various modes of Chou‘s pseudo amino acid composition were developed for improving the quality of predicting subcellular localization of apoptosis proteins based on their sequence information alone.
标签:
原文地址:http://www.cnblogs.com/biopy/p/4794191.html